Since 2011, the Leibniz Research Institute for Environmental Medicine (IUF) has been a member of the Leibniz Association. It researches environmentally induced diseases, particularly biological effects of environmental pollutants on the human body, mainly on skin, lungs and brain, but also other organs. The institute is divided into two research departments, one for environmentally induced aging processes and the other for environmentally induced immune disorders / immunotoxicology.
Prof. Dr. Ellen Fritsche is the head of the work group “Environmental Toxicological Risk Assessment and Human Sphere Models”, at the Leibniz Research Institute for Environmental Medicine (IUF), Heinrich Heine University Düsseldorf.
Work Group “Environmental Toxicological Risk Assessment and Human Sphere Models”, IUF, Düsseldorf. In the back row, third from left: Prof. Ellen Fritsche.
Photo: IUF
Since 2002, her special field has focussed on development neurotoxicity1 and dermatotoxicology. The main subject of the working group’s in vitro research are methods for replacing and complementing animal experiments on the one hand, research into molecular mechanisms of adverse2 effects of environmental noxae3 on human cells on the other.
In research toward developing complementary and replacement methods for animal experiments, especially the mechanism of relevant metabolic pathways in response to a potentially harmful substance is identified and analysed. These metabolic signal pathways are known as "Adverse Outcome Pathways" (AOPs). The most important task is to determine species-specific differences (between humans and animals) in these metabolic signal pathways. As replacing animal experiments is not yet technically feasible, the analysis of species-specific pathway differences should help to extrapolate4 the results of animal experiments to humans, thus improving risk assessment for humans.
The signal pathways currently being investigated in the Prof. Fritsche’s work group include the aryl hydrocarbon receptor5, the oestrogen receptor6, the thyroid receptor7 and the transcription factor Nrf28. As far as possible, functional, cell-biological endpoints are always correlated with the molecular endpoints9, usually in three-dimensional (3D) cell models, comprising primary cells10.
PhD student Jenny Baumann cultivates neurospheres in the incubator.
Image: IUF.
Aryl hydrocarbon receptor (AhR)
The aryl hydrocarbon receptor is a protein. However, in cytosol it is present as a protein complex with further proteins (1). In the case of contact with an exogenous substance (xenobiotic), the substance is bound to the AhR, which migrates to the nucleus and binds to a specific site of the DNA sequence, allowing the initiation of the production of a protein from the corresponding gene segment. Genes are read which encode proteins with oxidising and reducing properties (from the cytochrome P450 family), in particular CYP1A1 and CYP1B1. First, the proteins provide for a modification of the exogenous substance by installation of an oxygen atom (2). Thus the AhR is a kind of trigger for this process. However, even more metabolic enzymes involved in this process are influenced by AhR. The Ah receptor is not specialised, but is active in many different xenobiotics. Essentially, CYP1A1 mediates the activation of polyaromatic hydrocarbons to mutagens. In addition, CYP1A1 is also involved in the metabolism of oestradiol to 2-hydroxyoestradiol, whereas the metabolites of oestradiol are suspected of causing cancer (3). CYP1B1 has a similar function: it is the key enzyme in the metabolic activation of toxic environmental pollutants (e.g. polycyclic aromatic hydrocarbons such as benzo[a]pyrene) and endogenous substances such as oestrogenic hormones to ultimately carcinogenic metabolites (4).
Jenny Baumann monitors the automatic preparation of serial dilutions for chemical testing.
Image: IUF.
Oestrogen receptor
Oestrogen receptors are steroid receptors which are activated by the female hormone oestrogen. If such a receptor has bound an oestrogen, it combines with another oestrogen receptor to a so-called dimer complex and migrates into the nucleus further to the DNA, where a specific region of the genetic material is read out (5). Steroid hormones have a variety of different functions in mammals. They regulate differentiation, proliferation (growth and cell division), apoptosis (programmed cell death) and the functions of a large number of different cell types. Probably almost any tissue type is affected by at least one of the various steroid hormones (6).
One important context is the protection of skin against ageing due to UV radiation: oestrogen is able to release a growth factor from keratinocytes in the skin, thereby raising the levels of hyaluronic acid and versican V2 (a hyaluron-binding proteoglycan), which supports cell division (proliferation) and counteracts inflammatory processes (7). Hyaluronic acid is able to bind very large amounts of water relative to its mass, especially in connective tissue.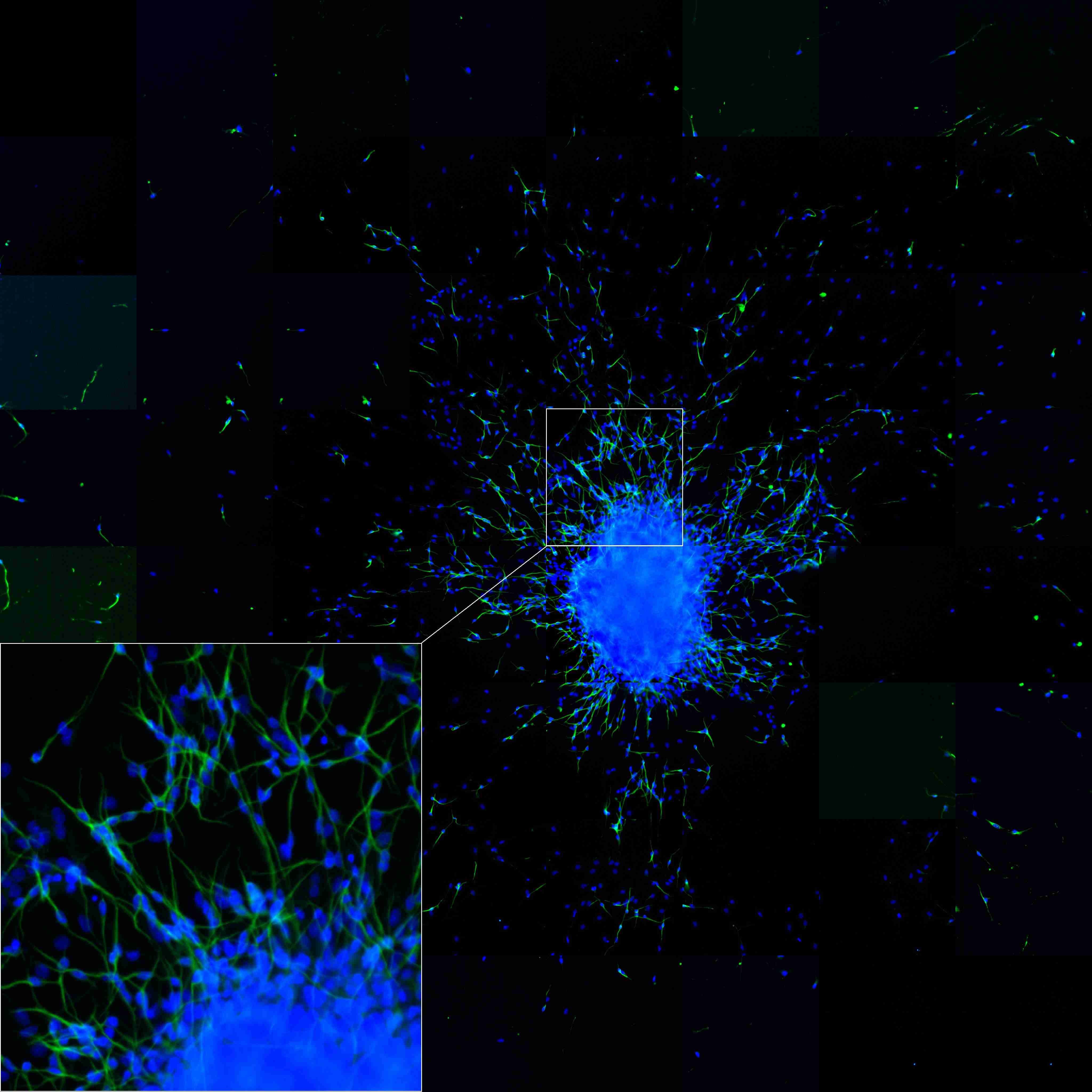
Neurospheres (see box, shown in blue) and incipient cell differentiation into nerve cells as well as their migration (bottom, left side). To be seen are cell bodies in a blue DAPI fluorescent dye, nerve fibres (axons) in green /yellow (coupled beta (III) tubulin antibodies in a green fluorescence).
Photo: IUF.
Neurospheres and NHNP cells
Similar to embryonic stem cells, neural stem cells can renew themselves and are indefinitely viable. However, they differ from the embryonic stem cells in terms of their differentiation potential, because they can develop only three dominant cerebral cell types, astrocytes, oligodendrocytes and neurons. Neural stem cells can be cultivated as single cell suspensions as well as in three-dimensional cell aggregates called neurospheres (8). Cultured in vitro they grow in a spherical shape (neurospheres). They can simulate the process of brain development (9).
NHNP cells are defined as “normal human neural progenitor cells” which are cultivated as neurospheres. As companies have specialised in breeding NHNPs they can also be purchased and used for testing purposes, for example for studying the developmental neurotoxic effects of substances. In Prof. Fritsche´s work group such a three-dimensional cell system has been developed from human neural progenitor cells. This method can be used for testing cosmetics, medicines or chemicals for their impairment of nerve cells.
M. Sc. Martin Schmuck measures the number of differentiated neurons from neurospheres, as well as neurite lengths.
Photo: IUF.
Three-dimensional primary cell systems best reflect metabolism
The choice of cell systems is a central topic of the working group, because on the one hand primary cells differ from immortalised cells11 or tumour cells in their molecular program, on the other hand cells grown in 3D organisation display other signal transduction pathways12 than cells grown completely on plastic surfaces in 2D. The group has also taken up this issue with regard to the xenobiotic metabolism pivotal to toxicology. It has been shown that 3D cell systems consisting of primary cells reflect the metabolism of the original organ most authentically.
To sum up, the research group of Prof. Fritsche investigates pathway-specific interferences of cellular functions by environmental pollutants in human and rodent 3D models. Such systems are to be used for detecting potential dangers or the protective effects of substances on the developing nervous system and the skin.
Thyroid hormone receptor
Through their contact with the blood vessels, astrocytes, a cell type in the grey and white matter of the brain, play an important role in the nutrition of nerve cells. For example, they transport thyroid hormones via the thyroid receptors into nerve tissue (10). Thyroid hormones are involved in growth, development and function of the central nervous system (11). The thyroid hormone 3,3',5-triiodo-L-thyronine (T3) produced by the thyroid gland affects both the differentiation and the maturing process of nerve cells (11). Under the influence of T3 in vitro, precursor cells terminate their proliferation and begin to differentiate. The hormone also promotes the maturation of the cells, their longitudinal growth and their branching. Thyroid dysfunction has a significant influence on the development of white matter in the central nervous system. The effect of T3 is mediated by the binding of the hormone to its thyroid receptor (TR). After the binding of the ligand (hormone) the TR acts as a transcription factor by binding to a regulatory acting DNA sequence thereby regulating gene expression (12).
Polychlorinated biphenyls (PCBs) affect brain development. With the NHNP neurosphere model of Prof. Fritsche´s working group, these processes can be studied accurately. As the precursor cells develop into astrocytes, oligodendrocytes and neurocytes, not only the metabolism of xenobiotics can be shown but also the signal transduction pathway of the thyroid hormone. For instance, experiments have shown that PCBs use the metabolic pathway of thyroid hormone showing effects similar to those of the thyroid hormone (13).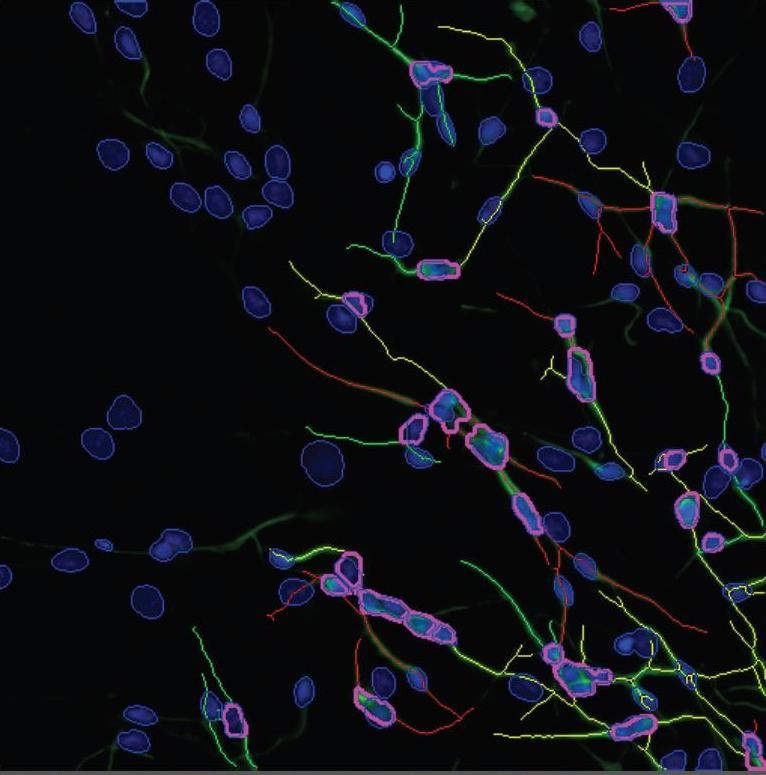
Neurosphere differentiation: NHNP (normal human neural progenitor) cells differentiate into neurocytes, astrocytes and oligodendrocytes. Shown here are neurocytes (stained with the marker ß (III) tubulin, green colour) and astrocytes (stained with the red marker GFAP).
Photo: IUF.
Transcription factor Nrf2
The transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) is responsible for the upregulation of the antioxidant response element (ARE)-mediated gene expression. Enzymes and proteins which are expressed by this Nrf2/ARE pathway possess chemically versatile cell protecting properties and form a defence against toxic metabolites and xenobiotics (14).
The aryl hydrocarbon receptor (AhR) is a transcription factor and binds to xenobiotic substances such as dioxins. Hereafter it moves from the cytosol to the nucleus in order to bind to a specific site on the DNA. The gene segment, which is then transcribed and translated into a protein, codes for enzymes of phase I and II (see box AhR). Many of the phase II enzymes are co-regulated with aid of the transcription factor Nrf2, the main regulator of antioxidant cell response. Therefore the two transcription factors cooperate in stress response against potentially harmful substances. The anti-inflammatory role of the Ah-receptor in the skin is traced to the activation of the cell protective Nrf2 system (15).
“...We want to understand the molecular differences between species”
InVitroJobs interviewed Prof. Dr. med. Ellen Fritsche on current developments in her research and an outlook on future developments.
InVitroJobs: How many staff are there in your work group in Düsseldorf?
Prof. Dr. med. Ellen Fritsche: We have 11 permanent employees and one or two masters students on annual rotation.
InVitroJobs: What questions are you currently investigating? What is the current stage of development?
Prof. Dr. med. Ellen Fritsche: At the moment we are concentrating on the molecular characterisation of species differences, with the quantitative detection of signal paths that lead to changes in cellular function. We have also begun generating reprogrammed human cells, from which unlimited numbers of different cell types can be differentiated without ethical concerns. I believe that working with such induced pluripotent stem cells (iPS cells)13 will be the basis of toxicology in the twenty-first century.
InVitroJobs: Can this reduce or replace animal experiments, and if so, which?
Prof. Dr. med. Ellen Fritsche: As alternative methods are currently not accepted for regulating chemicals in the area of (developmental) neurotoxicity testing, our tests could for instance be used for early in-house pre-screening14. This would reduce the number of animal experiments. Should the test be approved in the future for scientifically sound in vitro15 processes for regulatory purposes, our work is relevant to the OECD Guideline 42616 for developmental neurotoxicity or part of the Extended One-Generation Reproductive Toxicity Study17 (OECD TG 443).
InVitroJobs: Is the method currently in the optimisation process, in prevalidation18 or in validation19?
Prof. Dr. med. Ellen Fritsche: The method is currently in prevalidation, and funds for validation have been applied for.
InVitroJobs: Can the results of your research also be used for fundamental research?
Prof. Dr. med. Ellen Fritsche: I think that our work is also essential for fundamental research, for two reasons. On the one hand, how close the in vitro system used is to the in vivo situation is especially important for basic research, in order to be able to draw the right conclusions from the work. A tumour cell reacts differently in many ways to an exogenic stimulus than a “normal”, non-transformed cell. On the other hand, the translational aspect, that is, the extrapolation from animal data to humans via corresponding human and rodent in vitro systems (in accordance with the parallelogram approach; explanation further down in the interview), is also extremely important for basic research. This approach helps for instance to understand the significance of results from transgenic animals for humans. Such primary cells systems are also suitable for replacing certain work on transgenic animals, by knocking down21 or over-expressing22 genes by means of viral transduction20 in 3D systems. My work has established these techniques and we now also use them regularly for investigating questions related to fundamental research.
InVitroJobs: I can imagine that there are quite a few young scientists wanting to work with you.
Prof. Dr. med. Ellen Fritsche: I am very glad to say that this is indeed the case. Just this week I heard that a talented young scientist from Korea has been awarded a highly sought-after Leibniz-DAAD postdoc scholarship and will be working in our work group from the beginning of next year. This is the second time running that one of these scholarship holders has decided to join my work group. In addition, students from the Heinrich-Heine-University Düsseldorf regularly send me applications to complete their final papers in my work group.
InVitroJobs: You have been working on replacement methods for animal experiments since 2001. Has the research atmosphere improved or worsened since then?
Prof. Dr. med. Ellen Fritsche: My feeling is that the atmosphere has improved. On the one hand, this is due to the ever-increasing available data, which means that the path to success is continually becoming more clearly defined and that uncertainties become less. On the other hand, such US mammoth projects as ToxCast (16) and TT21C (17) contribute to a significant improvement in the disposition within Europe toward alternative test methods and modern concepts for risk assessment. The US is an absolute trailblazer in this respect.
InVitroJobs: What is your experience with regard to the differences between humans and animals, especially in cell cultures? What insights are there regarding the functional differences between animal and human cellular signal pathways?
Prof. Dr. med. Ellen Fritsche: My work group’s experiences demonstrate very clearly that there are species differences between humans and animals in the cellular pathways, whereby our experience is confined to differences in neural precursor cells between human and mouse or human and rat. It is interesting that a rat has just been generated that does not possess any aryl hydrocarbon receptors (AhR). The corresponding mouse has already existed for many years. The clinical phenotype of the AhR Knockout Rat is, however, distinctly different to that of the AhR Knockout Mouse, as our in vitro observations confirm. Why should humans be more similar to one rodent than two different rodent species are to one another? This is exactly what the American TT21C concept is based on, using human cells as test subjects. We are going one step further and want to understand the molecular differences between species, and use this information to improve risk assessment for humans in addition to the animal experiments which unfortunately have been unavoidable so far. Once the critical signal pathways for each cell type have been understood, it will be possible, in conjunction with the various mathematical modelling possibilities (such as quantitative structure-activity relationship23, pharmacokinetic modelling24), to replace more animal experiments.
InVitroJobs: At present there are still a lot of animals being used in research. Are the functional differences between humans and animals not regarded as being very significant?
Prof. Dr. med. Ellen Fritsche: Although these differences are known in fundamental research, the effects within the organism as a whole as opposed to cell cultures are generally treated with higher priority than the species differences between rodents and humans. In my opinion, however, both are important and thus valid.
InVitroJobs: Could one say the AhR signal pathway is one of the “pathways of toxicity”25 being sought after?
Prof. Dr. med. Ellen Fritsche: The AhR is a very ambivalent protein. On the one hand it mediates the toxicities of polycyclic aromatic hydrocarbons, UVB radiation and other noxae, and thus contributes to the development of skin tumours. On the other it also seems to be responsible for many functions of cell homoeostasis26, thus it also seems to participate in some way in the protection of cells. This seems to be a melange of cell type, ligand type and dosage. This is what makes AhR research so complicated – and so intriguing. But overall one can call the AhR signal a critical AOP (see above). Thus the insight that it is almost not expressed at all in the foetal human brain and also not functional is of great significance, especially with regard to its expression and functionality in the foetal rodent brain. I am always asked about the significance of the missing AhR expression in the foetal human brain. My assumption is that this absence of this receptor protects the developing human brain against PAH-induced oxidative stress, thus allowing it to develop undisturbed.
InVitroJobs: Even if the subject is complicated and convoluted, can you explain in a few simple sentences what the problem is in developing suitable replacement methods for animal toxicity experiments?
Prof. Dr. med. Ellen Fritsche: Of course the problems are many-layered, but I will try to reduce them to the most important aspects. The underlying problem is the in vitro/in vivo extrapolation. What do my in vitro results mean in vivo for the organism as a whole? On the one hand there is the kinetic problem, that is, the difficulty gauging the internal (intracellular) dose in vitro versus the organ dose in vivo. On the other hand there is the question of the in vitro endpoint. Is cytotoxicity an essential marker? Or are alterations in gene expression markers that allow good predictability of toxicity? If so, how does one distinguish adaptive from adverse alterations? I believe it is helpful to decide according to functional parameters, based on specific cell types. In addition, an in vitro test must be validated against the adequate correlating in vivo test. Human data are often rare, so that we have to refer to existing animal experiments. In light of the species differences we have discussed, however, it is not sensible to validate a human in vitro system against an animal experiment. Therefore, “proof of concept”27 should really achieved by validating the corresponding in animal vitro model against the animal experiment (for approval of the in vitro test), in order to then be able to gather data on the species differences between animal and human. My work group is pursuing this “parallelogram approach”, which will hopefully improve the acceptance of replacement methods in the long term.
InVitroJobs: How far is the development of suitable skin sensitisation tests? Can you say when suitable replacement methods for the endpoint “skin sensitisation” will be approved?
Prof. Dr. med. Ellen Fritsche: Recently I saw some very promising data from a large German company, and I am confident that approval of such methods in this field will take place in the near future.
InVitroJobs: What are your wishes for the future?
Prof. Dr. med. Ellen Fritsche: I would like to have sufficient funding to be able to use the example of neurospheres in a proof of concept to demonstrate that 3D rodent and human cultures using the classic parallelogram approach and taking AOPs into account are suitable for reliably predicting toxicities.
InVitroJobs: Thank you for the interview.
Glossary:
1 Development neurotoxicology: deals with the functional and morphological effects of exogenous substances on the developing nervous system, by exposure during pregnancy or the neonatal period leading to pathological brain changes.
2 adverse: harmful
3 environmental noxae: (med) substances or factors which exert a damaging (i.e. disease-causing) effect on an organism or a body organ.
4 extrapolation: a statistical approximation process. Conclusions regarding a development are drawn from collected data. The drawn conclusions are not fully verified (http://de.statista.com/statistik/lexikon/definition/54/extrapolation/)
5 aryl hydrocarbon receptor (AhR): The aryl hydrocarbon receptor is a protein (see box in text).
6 oestrogen receptor: a steroid receptor which is activated by the hormone oestrogen and then acts as a transcription factor ensuring that a specific gene segment is read out from the DNA and consequently translated into a protein (see box) (http://en.wikipedia.org/wiki/Oestrogen_receptor)
7 thyroid hormone receptor: The thyroid hormone 3,3',5-triiodo-L-thyronine (T3) produced by the thyroid gland affects both the differentiation and the maturation of nerve cells. Under influence of T3 in vitro progenitor cells terminate cell division and begin their differentiation. The effect of T3 is mediated by the binding of the hormone to its receptor (TR) (see box in text).
8 transcription factor Nrf2: The transcription factor Nrf2 is responsible for the upregulation of the antioxidant response element (ARE)-mediated gene expression (see box).
9 endpoint: study objective.
10 primary cells: primary cultures which grow directly after cell or tissue extraction. Usually they are not capable of dividing indefinitely.
11 immortalised cells: cell lines are cells of a tissue type that can breed indefinitely in culture. Using genetic engineering methods the cells are capable to divide indefinitely (immortalisation). Tissue spheroids: three-dimensional tissue beads, the chemical environment and the cell-cell contacts are different to those growing in shallow layers.
12 signal transduction pathway: a process by which cells respond to external stimuli, transform and transnit them to the interior of the cell. Here a variety of enzymes and secondary messengers are often involved (http://de.wikipedia.org/wiki/Signaltransduktion).
13 iPS (induced pluripotent stem cells): pluripotent stem cells which are generated by artificial re-programming of differentiated cells. The conversion is externally stimulated. For this purpose so-called transcription factors (proteins that bind to specific sites of the DNA) are used to turn on certain genes in the cells. iPS cells strongly resemble natural stem cells in many respects.
14 screening studies: Screening is a sifting test based on certain criteria. A screening study allows for example doctors and pharmaceutical companies to identify the most appropriate method for the detection of certain diseases or health conditions.
15 In vitro methods: in a test tube, tests or measurements conducted on living tissue outside of a living organism in an artificial environment, such as a Petri dish. By contrast, “in vivo” means procedures and experiments conducted in the living organism (animals).
16 OECD Guideline 426: European regulation of the procedure for the investigation of substance effects on development neurotoxicity. The animal most preferably to be used in this kind of experiments is the rat. Pregnant animals are administered the substance and their offspring are examined to determine development damages. The test guideline is not uncontroversial due to the species differences between humans and rats (18, 19).
17 extended one-generation study (OECD test guideline 443): In 2011 in Paris, the Organisation for Economic Cooperation and Development (OECD) adopted a regulation for testing chemicals for their potential developmental effects. Based on the “extended One Generation Reproductive Toxicity Study”, the number of animals used in experiments can be reduced by almost half compared to previous test numbers. The stressful animal tests do not have to be conducted on the second generation of animals because it has been shown that this provides no additional insights (20).
18 prevalidation study: collaborative study involving several independent test laboratories with the aim to determine whether the method is reliable and reproducible.
19 validation: Validation provides documented evidence that a process or a system fulfils the previously specified requirements (acceptance criteria) in a reproducible and practical manner (http://de.wikipedia.org/wiki/Validierung_ 28Pharmatechnik% 29%)
20 viral transduction: With the help of viral particles, genetic material is specifically introduced into living cells. The transport of DNA into a cell using a virus is known as transduction. (Http://de.wikipedia.org/wiki/Viraler_Vektor)
21 knock-down: by controlled incorporation of genetic foreign material with the help of viruses, a new gene sequence can be placed in the middle of an original gene sequence, so that this gene cannot be transcribed and translated into a protein.
22 over-expression: as a result of a genetic engineering method a particular gene is particularly strongly translated into a protein.
23 quantitative structure-activity relationship: a quantitative relationship between the pharmacological, chemical, biological and physical effects of a molecule and its chemical structure (http://en.wikipedia.org/wiki/Quantitative_structure%E2%80%93activity_relationship)
24 pharmacokinetic modelling: pharmacokinetics describes the entirety of all processes drug is subject to in the body. These include the uptake of the drug (absorption), the distribution in the body (distribution), the biochemical conversion and degradation (metabolism) and elimination (excretion). Additionally, before resorption, the release (liberation) of the drug from the dosage form can be significant. (http://de.wikipedia.org/wiki/Pharmakokinetik). The entirety of all available information should ultimately lead to the development of a metabolic sequence model for the prediction and risk assessment of substances.
25 pathway of toxicity (PoT): the metabolism pathway of a poison.
26 cell homoeostasis: physiological striving for equilibrium within the cell.
27 proof of concept: A project management concept, a milestone (point) at which the feasibility of a study is proven (http://de.wikipedia.org/wiki/Proof_of_Concept).
Literaturquellen:
(1) Jux, B. (2008): Die Bedeutung des Arylhydrocarbon Rezeptors für die Reifung und Funktion epidermaler Langerhans-Zellen in der Maus, Dissertation, Düsseldorf.
(2) http://de.wikipedia.org/wiki/Cytochrom_P450
(3) http://www.pharmagenomics.de/index.php/produkte/realtime-pcr-kits/cytochrome-p450/cyp1a1
(4) http://www.charite.de/forschungsberichte/FOB_2003-2005/deutsch/PJ/PJ15051.html
(5) http://de.wikipedia.org/wiki/Estrogenrezeptor
(6) Flötotto, T. (2001): Untersuchung alternativer Mechanismen der Genregulation
durch Östrogenrezeptor-Isoformen. Dissertation, Heinrich-Heine-Universität, Düsseldorf. http://docserv.uni-duesseldorf.de/servlets/DerivateServlet/Derivate-2152/152.pdf
(7) Röck, K. et al. (2012): Estradiol Protects Dermal Hyaloron/Versican Matrix during Photoaging by Release of Epidermal Growth Factor from Keratinocytes. J. Biol. Chem. 287 (24): 20056-69.
(8) Moors, M. (2007): Normale Humane Neurale Progenitorzellen als in vitro Modellsystem für entwicklungsneuro-toxikologische Untersuchungen: Molekulare und zellbiologische Charakterisierung. Inaugural-Dissertation Heinrich-Heine-Universität, Düsseldorf.
(9) Moors, M. et al. (2009): Human Neurospheres as Three-Dimensional Cellular Systems for Developmental Neurotoxicity Testing. Environmental Health Perspectives 117 (7): 1131-1138.
(10) Goncalves Trentin, A. (2006): Thyroid hormone and atsrocyte morphogenesis. Journal of Endocrinology 189: 189-197.
(11) König, S. & Moura Neto, V. (2002): Thyroid hormone actions on neural cells. Cell Mol Neurobiol. 22 (5-6): 517-544.
(12) Huber, K. (2005): Urspung, Entwicklung und Differenzierung von Oligodendrozyten. Dissertation Universität München. http://edoc.ub.uni-muenchen.de/4250/1/Huber_Katharina.pdf
(13) Fritsche, E., et al. (2005): Polychlorinated Biphenyls Disturb Differentiation of Normal Human Neural Progenitor Cells: Clue for Involvement of thyroid Hormone Receptors. Environmental Health Perspectives 113/7: 871-876.
(14) Deutsche Multiple Sklerose Gesellschaft http://www.dmsg.de/multiple-sklerose-forum/index.php?w3pid=msforum&kategorie=forum&tnr=8&mnr=61487
(15) Haarmann-Stemmann, T., Abel, J., Fritsche, E. & Krutmann, J. (2012): The AhR-Nrf2 pathway in keratinocytes: on the road to chemoprevention? J Invest Dermatol. 132 (1): 7-9. doi: 10.1038/jid.2011.359.
(16) ToxCast: http://www.epa.gov/ncct/toxcast/
(17) Tox21c: Tocixity Testing in the 21st Century. A Vision and a Strategy. Commitee on Toxicity Testing ans Assessment of Environmental Agents, National Research Council of the National Academies. http://www.nap.edu/catalog.php?record_id=11970
(18) http://www.oecd.org/dataoecd/20/52/37622194.pdf
(19) http://forums.alttox.org/index.php?PHPSESSID=3ecff1a21780010e29032a5949b4e506&topic=97.msg151#msg151
(20) http://forums.alttox.org/index.php?topic=702.msg1094#msg1094
Thursday, 12 July 2012 21:33
Working Group-a Portrait: Environmental Toxicological Risk Assessment and Models of Human Spheres Featured
InvitroJobs presents scientists and their innovative research in a regular feature called “Working Group – a Portrait”. We will focus on newly developed methods, their evaluation and their potential for reducing and where possible replacing animal experimentation according to the 3R principles of Russel & Burch (reduce, refine, replace).
In this issue we introduce the work group “Environmental Toxicological Risk Assessment and Human Sphere Models”, at the Leibniz Research Institute for Environmental Medicine (IUF), Heinrich Heine University Düsseldorf.




 Dr. rer. nat.
Dr. rer. nat. Menschen für Tierrechte - Tierversuchsgegner Rheinland-Pfalz e.V.
Menschen für Tierrechte - Tierversuchsgegner Rheinland-Pfalz e.V.