With the Animal Welfare Research Award, the German Federal Ministry of Food, Agriculture and Consumer Protection (BMELV) honours scientific work that especially contributes to the further development of pharmaco-toxicological test methods.
Dr. Landsiedel and his team have developed or co-developed three integrated test methods for local toxicity (irritation of the eyes and skin, as well as skin sensitisation. The award he receives today honours this achievement. So-called integrated test strategies are approaches that integrate different types of data and information with decision-making processes1. In addition to information from single tests and/or test batteries (i.e. graduated test systems), integrated test systems can also take into account approaches such as “weight of evidence” und exposure/population data for the final risk assessment of a substance1.
This year´s recipient of the Animal Welfare Research Award 2013 (from left to right: Dr. Peter Bleser, Federal Secretary of the BMELV, Dr. Robert Landsiedel, Dr. Susanne Kolle, Dr. Caroline Bauch, Prof. Dr. Ben van Raventzwaay.
Photo: Günter Friedmann-Marohn, BfR.
An assessment of local irritation, and skin and eye sensitisation must be conducted as part of safety evaluation within the framework of the EU REACh Directive for the production chemicals in excess of a tonne per year, but also plays a part in the production of pesticides or cosmetics. BASF has developed or co-developed seven methods in the area of eye irritation, chemical burns and skin irritation. Several of the methods have already been adopted into the OECD test guidelines; the others are still awaiting approval. BASF has incorporated the methods in so-called test strategies, and uses them routinely for testing chemicals. The laboratory for eye and skin toxicity implemented animal-free alternatives in 2009, and has not used any animal tests at all since 2012.
In vitro skin irritant tests
There are now two approved tests in the OECD test guidelines, the in vitro corrosion test (OECD 431) and the in vitro irritation test (OECD 439). The tests are based on the use of three-dimensional reconstructions of skin models. BASF was involved in the validation of these methods.
In vitro eye irritant tests
Eye irritation can be tested using the Bovine Cornea Opacity and Permeability Test (BCOP-Test; OECD 437). BASF played a pivotal part in the development and implementation of this method. The measuring instrument required for this test, an opacitometer, was not commercially available. In order to facilitate the worldwide use of this animal test-free method , BASF developed a device, which it provides to other laboratories at cost price.
Whereas the BCOP detects severe eye irritation, weaker irritation effects can be tested using human three-dimensional cornea models. This test is already used by BASF, so that the Draize test on rabbits can be done away with entirely.
An addition to the BCOP, there is a further test that can also be used as an alternative to rabbits, the Isolated Chicken Eye Test (ICE (OECD 438), which uses the eyes of slaughtered chicken. There is also the HCE (Human Corneal Epithelium-Test) and the HET-CAM Test (with fertilised chicken eggs). However, both these tests have not been approved by regulatory bodies.
Skin sensitisation
The allergenic potential of a substance is tested, with allergies being distinguished according to different types. In the case of skin sensitisation that occurs between 12 and 72 hours after skin contact with the substance,, researchers refer to allergy type 4. The allergy is based on a immune-mediated reaction. Persons often acquire such an allergy against preservatives, perfumes, colourants, or nickel or chrome compounds. Allergies can also be acquired to ingredients in cleaning agents and other consumer products. Once such an allergy has been acquired, it generally persists for the rest of that person’s life.
So far, the tests have been conducted on animals, with the goal of determining the potential for sensitisation. Traditionally, guinea pigs are used (Guinea Pig Maximization Test GMPT, OECD 406; or the Bühler-Test, OECD 406). In the Guinea Pig Maximization Test, the animals‘ skin is shorn and the substance administered together with adjuvants for a certain period of time, either intracutaneously or percutaneously. After a defined length of time, the researchers evaluate the redness and/or swelling of the skin caused by an allergic or inflammatory reaction.
![]()
Still a preferred species in skin sensitisation tests: guinea pigs.
Photo: StHuhn, Pixelio.
Within the framework of the 3R concept, the mural Local Lymph Node Assay (LLNA, OECD 429) is a refined alternative to the guinea pig tests. Fewer animals are used, and these are harmed less. The method investigates the immunological reaction in the animals’ lymph nodes. Nonetheless, it is invasive. It is based on the principle that sensitising substances trigger a primary lymphocyte proliferation (increased cell division) in the lymph nodes. After local administration of the test substance behind the animal‘s ears, the lymphocyte proliferation is measured with radioactive markers injected via the tail vein.
Humans can also be incorporated into the test series: In the Human Repeat Insult Patch Test (HPRIPT), the substance is repeatedly applied to the backs of volunteers at defined intervals and covered with plasters. Whether this test is ethical is subject to debate.
What characteristics must such a test have?
It must be able to identify the sensitisation potential of a chemical. This is sufficient for classification and labelling of a substance in accordance with the EU Regulation on classification, labelling and packaging of substances and mixtures (CLP). However, a complete risk evaluation requires the determination of its potency. Skin sensitisation is a complex process that cannot be covered by a single test, but rather requires an integrated test system comprising separate building blocks, in which several in vitro tests are able to reproduce the different aspects of sensitisation.
Several tests were already developed in 2010, however, the data from a single test were insufficient for the risk evaluation of a substance. Therefore, Dr. Landsiedel and his research colleagues developed a combined test that has been trialled in different European laboratories with regard to its reliability, transferability and reproducibility.
The test system comprises three tests: the Direct Peptide Reactivity Assay (DPRA); the human Cell Line Activation Test (h-CLAT), comprising a human cell line; and a keratinocyte activation assay (the KeratinoSensTM or the license-free LuSens-Assay developed by BASF).
1. Direct Peptide Reactivity Assay (DPRA)
Low molecular weight substances are themselves too small to be allergenic. They must first form a complex with the body’s own (endogenous) proteins. This mechanism that takes place within the body is emulated using the Direct Peptide Reactivity Assay (DPRA). Synthetic peptides with binding sites for cysteine and lysine simulate the endogenous proteins. By binding the test substance, the concentration of the synthetic peptides is reduced. The peptide reduction is measured and analysed, and acts as a gauge for the reaction between the test substance and the endogenous proteins.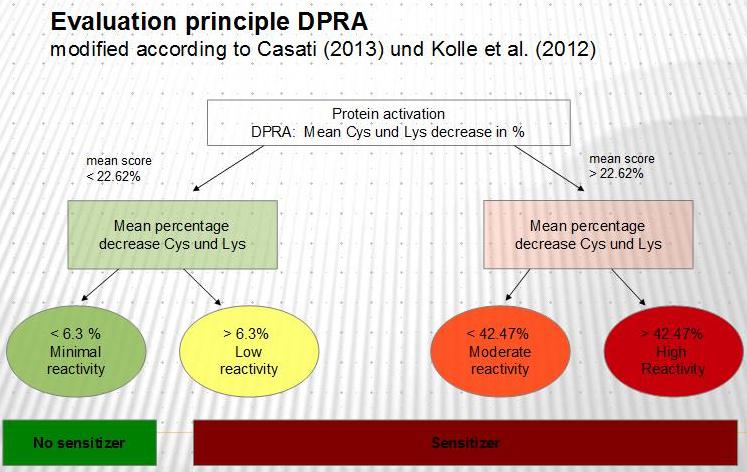
2. Human Cell Line Activation Test (hCLAT)
This measures the activation of cells in a culture. The monocytes used are precursors of a certain type of epidermal immune cells (Langerhans cells). After exposure to the test substance, the surface proteins (CD86, CD54) on the cells are measured using flow cytometry.
3. Keratinocyte activation assays
The third test assays a certain signal pathway within the cells, using immortalised human keratinocytes (specialised epidermal cells). This principle is based upon the fact that most allergens activate a particular signal pathway, Nrf2-Keap1-ARE. A reporter gene was built into the keratinocytes so as to be able to recognise signal pathway activation following exposure to a test substance using luminometry (A luciferase gene was inserted using the Antioxidant Response Element [ARE] to monitor transcription).
InVitroJobs spoke with the award recipient shortly before the award ceremony.
InVitroJobs:
We would like to congratulate you and your team. How do you feel today upon receiving this honour?
Dr. Landsiedel:
The whole team is very pleased about this recognition for their work. The greatest success is most certainly the fast that we can actually test a whole group of effects – the entire local toxicity – without using any animal tests, thanks to years of pragmatic development; and that we have in fact implemented this in the routine testing of chemicals. Today’s award is a further very visible acknowledgement of that fact.
InVitroJobs:
With your alternative labs, you are involved in developing many replacement methods. Can you tell us more specifically which of your developments is being honoured today?
Dr. Landsiedel:
The development of several methods that we have partly combined in test strategies to be able to test all endpoints of local toxicity without using animal tests; that is: skin irritation, skin burns, eye irritation and skin sensitisation.
InVitroJobs:
Is the Bovine Cornea Opacity and Permeability Test (or BCOP test), which has already been validated, widely used as a replacement for the eye irritation test on rabbits? There are still companies that conduct the test on rabbits, and I have read that the BCOP test still doesn’t count as a complete replacement for the eye irritation test. What is the reason for that? Are several tests necessary?
Dr. Landsiedel:
The BCOP was adopted by the OECD into a test guideline (no. 437). The test can detect severe eye irritation. In the summer of this year, the test guideline was revised, and now it is possible to rule out all kinds of eye irritation. We at BASF use EpiOcular, a corneal model using human cells, for the area between no irritation and severe eye irritation, i.e. for testing non-severe levels of irritation.
There are also substances – many formulations, for instance – that cannot be tested using the BCOP. Here further adaptation of the methods is necessary.
InVitroJobs:
International demand for you opacitometer has been considerable, and there has also been media interest. Are the Chinese now also using the apparatus?
Dr. Landsiedel:
So far we have delivered some 20 units. Parallel to that, labs are being trained in the correct implementation of the BCOP. The training courses are conducted by our partner in the USA, the Institute for In Vitro Science (IIVS). We have also held courses in China, but I can’t tell whether or how the BCOP is already used in China.
InVitroJobs:
How long did it take to develop the ITS far enough to be able to submit it to the ECVAM?
Dr. Landsiedel:
We tested 14 methods in total for the test strategy for skin sensitisation, and finally selected four methods as being suitable. We used three of these methods to develop a test strategy that predicts skin sensitisation just as well as the Local Lymph Node Assay (LLNA) does, which has been the ‘standard’ animal test so far. The development took us about five years, plus the work external partners invested in the methods our strategy is built on.
InVitroJobs:
How long will the approval/adoption into OECD test guidelines take?
Dr. Landsiedel:
I can’t say for sure. Going by current development and validation of the methods, I assume and hope that will happen in 2014.
We have also submitted the test strategy for validation to ECVAM. However, ECVAM has announced a test strategy for 2016 at the earliest. That is very late for most of the substances subject to REACH.
InVitroJobs:
What are you working on right now?
Dr. Landsiedel:
At the moment we are working on two aspects of skin sensitisation. How can we not only tell not only whether a substance is skin-sensitising but also how potent the sensitisation is? And how can we test substances that can’t be dissolved in cell culture media? In addition to that, we spent a long time investigating the more complex toxicological endpoints: reprotox and systemic toxicity. That will be our focus in the years to come.
InVitroJobs:
Thank you very much for speaking with us.
1 Integrated Testing Strategies & Risk Assessment (http://www.alttox.org/ttrc/emerging-technologies/its/)




 Dr. rer. nat.
Dr. rer. nat. Menschen für Tierrechte - Tierversuchsgegner Rheinland-Pfalz e.V.
Menschen für Tierrechte - Tierversuchsgegner Rheinland-Pfalz e.V.