The annual EURL ECVAM report (1) provides regular updates on progress in the development, validation, and regulatory acceptance of non-animal methods and their use, as well as the promotion of education and training programs.
In 2020, projects dealt with the development of case studies on substance groupings and read-across. ECVAM also identified the optimal combination of methods in integrated testing strategies (IATA) for systemic toxicity tests, and developed methods to improve the identification of endocrine-disrupting chemicals.
The European Commission agreed that new methods to replace animal testing - also called New Approach Methods (NAMs) - are urgently needed - innovative and more efficient ways of safety testing and chemical risk assessment that do not depend on animal testing should be found for some 90,000 chemicals on the EU market, says the ECVAM report.
As part of the Greal Deal (Chemicals Strategy for Sustainability for a Pollutant-Free Environment), the compass is set up: to necessary innovation in chemical safety testing and risk assessment to reduce reliance on animal testing, but also to improve the quality and efficiency of chemical hazard and risk assessments and to accelerate these assessments.(2)
Of course, one can critically note that all that is being talked about here is a reduction in animal testing (In 2017, chemical testing alone in the EU consumed 11% of the 2.19 million animals used in regulatory testing. Regulatory animal testing used 23% of all animals). However, there is a mention of the obligation to innovate, of independence from animal testing. It is clearly acknowledged, that animal testing does not lead to the necessary quality and is inefficient.
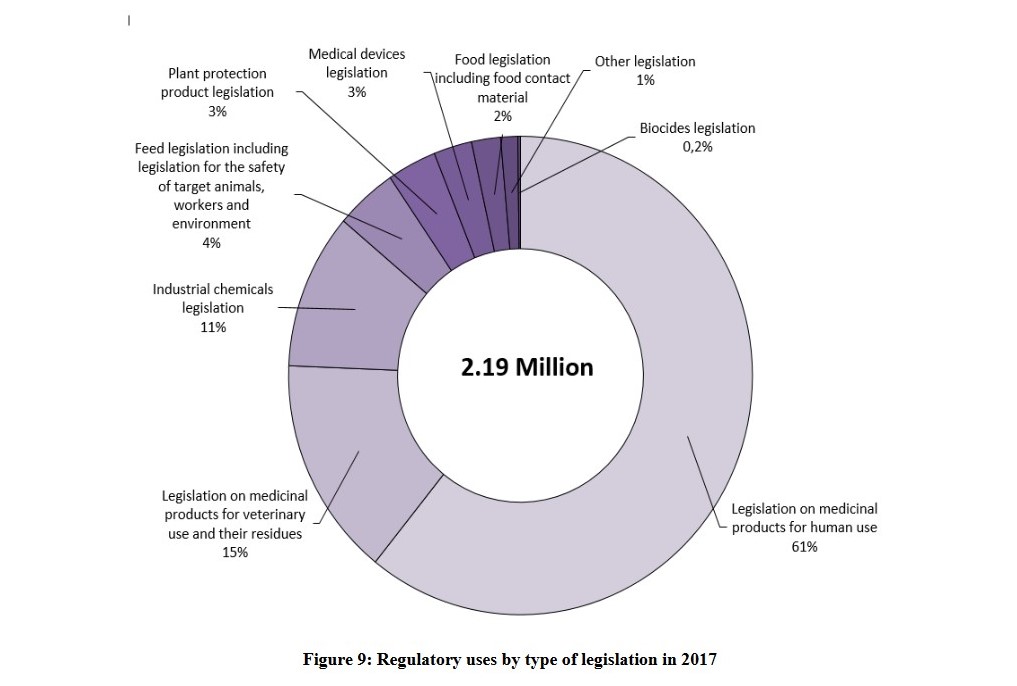
Source: European Commission (2019). 2019 report on the statistics on the use of animals for scientific purposes in the Member States of the European Union in 2015-2017. COM(2020) 16 final. https://ec.europa.eu/info/sites/info/files/com-2020-16-f1-en-main-part-1.pdf
ECVAM has also investigated methods suitable to evaluate endocrine disrupting substances, e.g. thyroid disrupting chemicals, and evaluated and validated new methods in skin and respiratory sensitization, genotoxicity as well as fish toxicity.
However, the validation authority has evaluated not only methods for chemical testing, but also those for vaccine production. Several non-animal methods for vaccine safety and potency testing were evaluated as part of the EU Vac2Vac project. For batch testing, ECVAM coordinated a guidance document for transitioning from an animal-based quality control strategy to an animal-free strategy based on consistency testing. ECVAM also launched a project to avoid duplication of animal testing. This will be done by making better use of data from animal studies. Other areas of work can be found in the ECVAM report.
The research was funded by the EU's Horizon 2020 program with the participation of numerous collaborative partners.
Sources and further information:
(1) EURL ECVAM (2021). Non-animal Methods in Science and Regulation. https://ec.europa.eu/jrc/en/publication/
(2) European Commission (2020). Communication from the Commission to the European Parliament, the European Council, the European Economic and Social Committee and the Committee of the Regions. COM(2020) 667 final. https://eur-lex.europa.eu/




 Dr. rer. nat.
Dr. rer. nat. Menschen für Tierrechte - Tierversuchsgegner Rheinland-Pfalz e.V.
Menschen für Tierrechte - Tierversuchsgegner Rheinland-Pfalz e.V.