Auf dieser Seite führt InVitroJobs eine Rubrik zum Thema „zukunftsweisende Entwicklungen“. Darunter sind Technologien zu verstehen, die geeignet sein könnten, in Zukunft den Tierverbrauch auf einem bestimmten Gebiet abzulösen.
Bildgebende Verfahren könnten an der einen oder anderen Stelle kognitionswissenschaftliche Versuche der Grundlagenforschung, z. B. solche mit nicht-humanen Primaten, durch humanspezifische, nicht-invasive Meßmethoden ersetzen. Sie erhalten hier Informationen in Form von Literaturquellen, die zum Abstract führen.
Für den Einsatz als Ersatzverfahren zu Tierversuchen sind diese vorgestellten Verfahren jedoch noch nicht hinreichend erprobt. Bislang sind die Technologien entwickelt worden, jedoch ist nicht bekannt, ob sie auf den Sachverhalt eines Tierversuchs übertragen worden sind. Es liegen derzeit noch keine Studien dazu vor, es sind keine Evaluierungen oder Validierungen bekannt.
Foto: Kasuga Huang.
1. Bildgebende Verfahren
ALI, A., ABOULEILA, Y., AMER, S., FURUSHIMA, R., EMARA, S., EQUIS, S., COTTE, Y. & MASUJIMA, T. (2016). Quantitative Live Single-cell Mass Spectrometry with Spatial Evaluation by Three-Dimensional Holographic and Tomographic Laser Microscopy. ANALYTICAL SCIENCES 32/2: 125-127.
Aramesh, M., Forró, C., Dorwling-Carter, L., Lüchtefeld, I., Schlotter, T., Ihle, S. J., Shorubalko, I., Hosseini, V., Momotenko, D., Zambelli, T., Klotzsch, E. & Vörös, J. (2019). Localized detection of ions and biomolecules with a force-controlled scanning nanopore microscope. Nature Nanotechnology, DOI: 10.1038/s41565-019-0493-z
Azarkh M, Bieber A, Qi M, Fischer JWA, Yulikov M, Godt A, Drescher M (2019). Gd(III)–Gd(III) Relaxation-Induced Dipolar Modulation Enhancement for In-Cell Electron Paramagnetic Resonance Distance Determination. J. Phys. Chem. Lett. 10: 1477–1481.
Bisdas S, Lá Fougere C & Ernemann U (2015): Hybrid MR-PET in Neuroimaging. Clin Neuroradiol. 2015 Oct; 25 Suppl 2:275-81. doi: 10.1007/s00062-015-0427-6. Epub 2015 Jul 31.
Bouchard, M. B., Voleti, V., Mendes, C. S., Lacefield, C., Grueber, W. B., Mann, R. S., Bruno, R. M. & Hillman, E. C. M. (2015): Swept confocally-aligned planar excitation (SCAPE) microscopy for high-speed volumetric imaging of behaving organisms. Nature Photonics, DOI: 10.1038/nphoton.2014.323
Chang BJ, Perez Meza VD, Stelzer EHK (2017): csiLSFM combines light-sheet fluorescence microscopy and coherent structured illumination for a lateral resolution below 100 nm. Proc Natl Acad Sci USA, 114 (19): 4869-4874. (2017 May 9). Epub 2017 Apr 24.
Cherry, SR, Jones, T, Karp, JS, Qi, J, Moses, WW, Badawi, RD (2018): Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J Nucl Med. 59 (1): 3-12. doi: 10.2967/jnumed.116.184028.
Chhatwal, J. P., Schultz, A. P., Johnson, K., Benzinger, T. L. S., Jack, Jr., C., Ances, B. M., Sullivan, C. A., Salloway, S. P., Ringman, J. M., Koeppe, R. A., Marcus, D. S., Thompson, P., Saykin, A. J., Correia, S., Schofield, P. R., Rowe, C. C., Fox, N. C., Brickman, A. M., Mayeux, R., McDade, E., Bateman, R., Fagan, A. M., Goate, A. M., Xiong, C., Buckles, V. D., Morris, J., C. & Sperling, R. A. (2013): Impaired default network functional connectivity in autosomal dominant Alzheimer disease. Neurology 81. DOI 10.1212/WNL.0b013e3182a1aafe
Choi, Woo June & Wang, Ruikang K. (2015): Swept-source optical coherence tomography powered by a 1.3-μm vertical cavity surface emitting laser enables 2.3-mm-deep brain imaging in mice in vivo. J. Biomed. Opt. 20(10), 106004 (Oct 08, 2015). doi:10.1117/1.JBO.20.10.106004
Chojnacki, J., Staudt, T., Glass, B., Bingen, P., Engelhardt, J., Anders, M., Schneider, J., Müller, B., Hell, S. W. & Kräusslich, H.-G. (2012): Maturation-Dependent HIV-1 Surface Protein Redistribution Revealed by Fluorescence Nanoscopy. Science 338/6106: 524-528.
Connolly, C. G., Wu, J., Ho, T. C., Hoeft, F., Wolkowitz, O. Eisendrath, S., Frank, G., Hendren, R., Max, J. E., Paulus, M. P., Tapert, S. F., Banerjee, D., Simmons, A. N. & Yang, T. T. (2013): Resting-State Functional Connectivity of Subgenual Anterior Cingulate Cortex in Depressed Adolescents. Biol. Psychiatry.
Dahmardeh, M., Mirzaalian Dastjerdi, H., Mazal, H. et al. (2023). Self-supervised machine learning pushes the sensitivity limit in label-free detection of single proteins below 10 kDa. Nat Methods 20, 442–447.
de Winkel, K.N., Nesti, A., Ayaz, H. & Bülthoff, H. H. (2017): Neural correlates of decision making on whole body yaw rotation: an fNIRS study. Neuroscience Letters, Available online 13 June 2017
Derix, J., Yang, S., Lüsebrink, F., Fiederer, L. D. J., Schulze-Bonhage, A., Aertsen, A., Speck, O. and Ball, T. (2014): Visualization of the amygdalo–hippocampal border and its structural variability by 7T and 3T magnetic resonance imaging. Hum. Brain Mapp. Early View (Online Version of Record published before inclusion in an issue). DOI: 10.1002/hbm.22477
Downing, P., Liu, J., & Kanwisher, N. (2001): Testing cognitive models of visual attention with fMRI and MEG. Neuropsychologia, 39/12: 1329-1342.
EMPA (2022). Miniaturized Quantum Dot Infrared Spectrometer.
Espy, M., Matlachov, A., Volegov, P., Mosher, J.C., & Kraus, R.H., Jr. (2005): SQUID-based simultaneous detection of NMR and biomagnetic signals at ultra-low magnetic fields. IEEE Trans. Appl. Supercond., 15: 635-639.
Gambarotto, D., Zwettler, F. U., Guennec, M. L., Schmidt-Cernohorska, M., Fortun, D., Borgers, S., Heine, J., Schloetel, J.-G., M., Reuss, Unser, M., E. S., Boyden, Sauer, M., Hamel, V. & Guichard, P. (2019). Imaging cellular ultrastructures using expansion microscopy (U-ExM). Nature Methods 16: 71-74.
Goulielmakis, E., Lakhotia, H., Kim, H-.Y., Zhan, M., Hu, S. & Meng, S. (2020). Laser picoscopy of valence electrons in solids. Nature 583: 55–59.
Gwosch KC, Pape JK, Balzarotti F, Hoess P, Ellenberg J, Ries J, Hell SW (2020). MINFLUX nanoscopy delivers 3D multicolor nanometer resolution in cells. Nature Methods.
Harrison, Ian F, Bernard Siow, Aisha B Akilo, Phoebe G Evans, Ozama Ismail, Yolanda Ohene, Payam Nahavandi, David L Thomas, Mark F Lythgoe & Jack A Wells (2018). Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with DTI MRI. eLife 2018;7:e34028 doi: 10.7554/eLife.34028.
Haynes, J.D. & Rees, G. 2005. Predicting the orientation of invisible stimuli from activity in human primary visual cortex. Nat.Neurosci., 8/5: 686-691.
Hipp, L., Beer, J., Kuchler, O., Reisser, M., Sinske, D., Michaelis, J., Gebhardt, J.C.M. & Knöll, B. (2019). Single-molecule imaging of the transcription factor SRF reveals prolonged chromatin-binding kinetics upon cell stimulation. Proc Natl Acad Sci USA, 116(3): 880-889.
Höfner, N., Albrecht, H. H., Cassara, A. M., Curio, G., Hartwig, S., Haueisen, J., Hilschenz, I., Korber, R., Martens, S., Scheer, H. J., Voigt, J., Trahms, L., & Burghoff, M. (2011): Are brain currents detectable by means of low-field NMR? A phantom study. Magn Reson. Imaging 29/10: 1365-1373.
Kaiplavil, Sreekumar & Mandelis, Andreas (2014): Truncated-correlation photothermal coherence tomography for deep subsurface analysis. Nature Photonics. doi:10.1038/nphoton.2014.111
Kamitani, Y. & Tong, F. (2006): Decoding seen and attended motion directions from activity in the human visual cortex. Curr.Biol, 16/11: 1096-1102.
Karampinos, Dimitrios C., Claussnitzer, Melina & Hauner, Hans (2022): Transcriptome and fatty-acid signatures of adipocyte hypertrophy and its non-invasive MR-based characterization in human adipose tissue. EBioMedicine.
Kauert, D.J., Madariaga-Marcos, J., Rutkauskas, M. et al. The energy landscape for R-loop formation by the CRISPR–Cas Cascade complex. Nat Struct Mol Biol 30, 1040–1047 (2023). https://doi.org/10.1038/s41594-023-01019-2
Klippel, S., Döpfert, J., Jabadurai Jayapaul, J., Kunth, M., Rossella, F., Schnurr, M., Witte, C., Freund, C. & Schröder, L. (2013: Cell tracking with Caged Xenon: Using Cryptophanes as MRI Reporters upon Cellular Internalization. (Epub ahead of print) DOI:10.1002/anie.201307290
König, K & Ostendorf, A (Eds.): Optically Induced Nanostructures. Biomedical and Technical Applications. Verlag DeGruyter (2015).
Kraus, R. H., Jr., Volegov, P., Matlachov, A., & Espy, M. (2008): Toward direct neural current imaging by resonant mechanisms at ultra-low field. Neuroimage., 39/1: 310-317.
Lavery, L., Merkle, A. & Gelb, J. (2015). 3D X-ray Microscopy: A New High Resolution Tomographic Technology for Biological Specimens. Microsc. Microanal. 21 (Suppl 3).
Le Bihan, D., Mangin, J.-F., Poupon, C., Clark, C. A., Pappata, S., Molko, N., & Chabriat, H. (2001): Diffusion Tensor Imaging: Concepts and Applications. Journal of Magnetic Resonance Imaging. 13: 534–546.
Lee, M. H., Smyser, C. D. & Shimony, J. S. (2012): Resting-State fMRI: A Review of Methods and Clinical Applications. AJNR Am. J. Neuroradiol. 10.3174/ajnr.A3263
Lee, T, Cai, LX, Lelyveld, VS, Aviad Hai & Alan Jasanoff (2014): Molecular-Level Functional Magnetic Resonance Imaging of Dopaminergic Signaling. Science 344: 533-535.
Li, Y., et al. (2020). Multifocal photoacoustic microscopy using a single-element ultrasonic transducer through an ergodic relay. Light: Science & Applications. doi.org/10.1038/s41377-020-00372-x.
Liangzhong Xiang, Bo Wang, Lijun Ji & Huabei Jiang (2013): 4-D Photoacoustic Tomography. Scientific Reports 3 : 1113, DOI: 10.1038/srep01113.
Liu, T.-L., Upadhyayula, S., Milkie, D. E., Singh, V., Wang, K., Swinburne, I. A., Mosaliganti, K. R., Collins, Z. M., Hiscock, T. W., Shea, J., Kohrman, A. Q., Medwig, T. N., Dambournet, D., Forster, R., Cunniff, B., Ruan, Y., Yashiro, H., Scholpp, S., Meyerowitz, E. M., Hockemeyer, D., Drubin, D. G., Martin, B. L., Matus, D. Q., Koyama, M., Megason, S. G., Kirchhausen, T. & Betzig, E. (2018). Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science 20, Vol. 360, Issue 6386, eaaq1392.
V. Loconte, J.-H. Chen, M. Cortese, A. Ekman, M. A. Le Gros, C. Larabell, R. Bartenschlager, V. Weinhardt; "Using soft X-ray tomography for rapid whole-cell quantitative imaging of SARS-CoV-2-infected cells"; Cell Reports Methods; Vol. 1, Issue 7, 22 November 2021, 100117
Loretz, M., Rosskopf, T., Boss, J.M., Pezzagna, S., Meijer, J. & C. L. Degen (2014): Single-proton spin detection by diamond magnetometry. Science Express Reports. doi: 10.1126/science.1259464
Mahamid, J., Pfeffer, S., Schaffer, M., Villa, E., Danev, R., Kuhn-Cuellar, L., Förster, F., Hyman, A. A., Plitzko, J. M., Baumeister, W. (2016): Visualizing the molecular sociology at the HeLa cell nuclear periphery. Science 351 (6276): 969-972. DOI: 10.1126/science.aad8857
Y. Matsumoto, Y. Asao, A. Yoshikawa, H. Sekiguchi, M. Takada, M. Furu, S. Saito, M. Kataoka, H. Abe, T. Yagi, K. Togashi & M. Toi (2018). Label-free photoacoustic imaging of human palmar vessels: a structural morphological analysis. Scientific Reports 8:786, DOI:10.1038/s41598-018-19161-z
Jacqueline L. S. Milne, Mario J. Borgnia, Alberto Bartesaghi, Erin E. H. Tran, Lesley A. Earl, David M. Schauder, Jeffrey Lengyel, Jason Pierson, Ardan Patwardhan & Sriram Subramaniam (2013). Cryo-electron microscopy: A primer for the non-microscopist. FEBS J. 280 (1): 28–45. doi:10.1111/febs.12078
-> Neu: Nanda, S., Calderon, A., Sachan, A. et al. Rho GTPase activity crosstalk mediated by Arhgef11 and Arhgef12 coordinates cell protrusion-retraction cycles. Nat Commun 14, 8356 (2023). https://doi.org/10.1038/s41467-023-43875-y
Nielles-Vallespin, S., Mekkaoui,C., Gatehouse, P., Reese, T. G., Keegan, J., Ferreira, P. F., Collins, S., Speier, P., Feiweier, T., de Silva, R., Jackowski, M. P., Pennell, D. J., Sosnovik, D. E. & Firmin, D. (2013): In Vivo Diffusion Tensor MRI of the Human Heart: Reproducibility of Breath-Hold and Navigator-Based Approaches. Magnetic Resonance in Medicine. 70: 454–465.
Mihajlo Novakovic, Gregory L. Olsen, György Pintér, Daniel Hymon, Boris Fürtig, Harald Schwalbe, Lucio Frydman: A 300-fold enhancement of imino nucleic acid resonances by hyperpolarized water provides a new window for probing RNA refolding by 1D and 2D NMR, PNAS, 16. Januar 2020
Jasmin K. Pape, Till Stephan, Francisco Balzarotti, Rebecca Büchner, Felix Lange, Dietmar Riedel, Stefan Jakobs, Stefan W. Hell (2020). Multicolor 3D MINFLUX nanoscopy of mitochondrial MICOS proteins. Proceedings of the National Academy of Sciences USA; 11. August 2020
Patching, Simon G. (2014): Surface plasmon resonance spectroscopy for characterisation of membrane protein–ligand interactions and its potential for drug discovery. Biochimica et Biophysica Acta 1838: 43–55.
Miguel A. Pleitez, Asrar Ali Khan, Alice Soldà, Andriy Chmyrov, Josefine Reber, Francesca Gasparin, Markus R. Seeger, Benedikt Schätz, Stephan Herzig, Marcel Scheideler & Vasilis Ntziachristos (2019). Label-free metabolic imaging by mid-infrared optoacoustic microscopy in living cells. Nature Biotechnology.
-> Neu: Reznik D, Trampel R, Weiskopf N, Witter MP, Doeller CF. (2023). Dissociating distinct cortical networks associated with subregions of the human medial temporal lobe using precision neuroimaging. Neuron. Jun 23: S0896-6273(23)00402-6. doi: 10.1016/j.neuron.2023.05.029. Epub ahead of print. PMID: 37390820.
Schindler, A. & Bartels, A. (2013): Parietal Cortex Codes for Egocentric Space beyond the Field of View. Current Biology 23, 1–6. http://dx.doi.
Schneider, J., Zahn, J., Maglione, M., Sigrist, S. J., Marquard, J., Chojnacki, J., Kräusslich, H.-G., Sahl, S. J., Engelhardt, J. & Hell, S. W.
(2015): Ultrafast, temporally stochastic STED nanoscopy of millisecond dynamics. Nature Methods 2015, 10.1038/nmeth.3481
Oliver Schoppe, Chenchen Pan, Javier Coronel, Hongcheng Mai, Zhouyi Rong, Mihail Ivilinov Todorov, Annemarie Müskes, Fernando Navarro, Hongwei Li, Ali Ertürk, Bjoern H. Menze (2020). Deep learning-enabled multi-organ segmentation in whole-body mouse scans. Nature communications.
Shibata, M., Uchihashi, T., Ando, T. & Yasuda, R. (2015): Long-tip high-speed atomic force microscopy for nanometer-scale imaging in live cells. SCIENTIFIC REPORTS 5: 8724, DOI: 10.1038/srep08724.
Heinz, A., Siessmeier, T., Wrase, J., Hermann, D., Klein, S., Grusser, S.M., Flor, H., Braus, D.F., Buchholz, H.G., Grunder, G., Schreckenberger, M., Smolka, M. N., Rosch, F., Mann, K., & Bartenstein, P. (2004): Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry, 161/10: 1783-1789.
-> Neu: Speiser, A., Müller, LR., Hoess, P. et al. Deep learning enables fast and dense single-molecule localization with high accuracy. Nat Methods (2021). https://doi.org/10.1038/s41592-021-01236-x
Stirman, JN, Smith, IT, Kudenov, MW & Smith, SL (2016): Wide field-of-view, multi-region, two-photon imaging of neuronal activity in the mammalian brain. Nature Biotechnology, advance online publication, doi:10.1038/nbt.3594.
Stratis Tzoumas, Antonio Nunes, Ivan Olefir, Stefan Stangl, Panagiotis Symvoulidis, Sarah Glasl, Christine Bayer, Gabriele Multhoff & Vasilis Ntziachristos (2016): Eigenspectra optoacoustic tomography achieves quantitative blood oxygenation imaging deep in tissues. Nature Communications 7, Article number: 12121 doi:10.1038/ncomms12121.
-> Neu: Sunbul, M., Lackner, J., Martin, A. et al. (2021). Super-resolution RNA imaging using a rhodamine-binding aptamer with fast exchange kinetics. Nat Biotechnol (2021). https://doi.org/10.1038/s41587-020-00794-3
Aleksander Szczurek, Ludger Klewes, Jun Xing, Amine Gourram, Udo Birk, Hans Knecht, Jurek W. Dobrucki, Sabine Mai & Christoph Cremer (2017). Imaging chromatin nanostructure with binding-activated localization microscopy based on DNA structure fluctuations. Nucleic Acids Research 45, No. 8 e56. doi: 10.1093/nar/gkw1301.
Thunemann M, Schörg BF, Feil S, Lin Y, Voelkl J, Golla M, Vachaviolos A, Kohlhofer U, Quintanilla-Martinez L, Olbrich M, Ehrlichmann W, Reischl G, Griessinger CM, Langer HF, Gawaz M, Lang F, Schäfers M, Kneilling M, Pichler BJ, Feil R. (2017): Cre/lox-assisted non-invasive in vivo tracking of specific cell populations by positron emission tomography. Nature Communications. DOI: 10.1038/s41467-017-00482-y.
Tong, F. Harrison S. A., Dewey, J. A., Kamitani, Y (2013): Relationship between BOLD amplitude and pattern classification of orientation-selective activity in the human visual cortex. NeuroImage 63 (2012) 1212–1222.
Andres Flores Valle & Johannes D. Seelig (2019). Two-photon Bessel beam tomography for fast volume imaging. Optics Express 12147, 27/9. https://doi.org/10.1364/OE.27.012147
Vraka, C., Pichler, V., Pfaff, S., Balber, T., Hacker, M., Mitterhauser, M., Wadsak, W., Philippe, C. (2019). Technical aspect of the automated synthesis and real-time kinetic evaluation of [11C] SNAP-7941. Jove 146, 28.04.2919, doi: 10.3791/59557.
Wehrl, H. F., Hossain, M., Lankes, K., Liu, C.-C., Bezrukov, I., Martirosian, P., Schick, F., Reischl, G. & Pichler, B. J. (2013): Simultaneous PET-MRI reveals brain function in activated and resting state on metabolic, hemodynamic and multiple temporal scales. Nature Medicine.
Martin Weigert, Uwe Schmidt, Tobias Boothe, Andreas Müller, Alexandr Dibrov, Akanksha Jain, Benjamin Wilhelm, Deborah Schmidt, Coleman Broaddus, Siân Culley, Mauricio Rocha-Martins, Fabián Segovia-Miranda, Caren Norden, Ricardo Henriques, Marino Zerial, Michele Solimena, Jochen Rink, Pavel Tomancak, Loic Royer, Florian Jug & Eugene W. Myers (2018). Content-aware image restoration: pushing the limits of fluorescence microscopy. Nature Methods 15: 1090–1097.
Weinstein J., Aviv Regev & Feng Zhang (2019). DNA microscopy: optics-free spatio-genetic imaging by a stand-alone chemical reaction. Cell. Online June 20, 2019. DOI: 10.1016/j.cell.2019.05.019
Weizenecker, J., Gleich, B., Rahmer, J., Dahnke, H. & Borgert, J. (2009): Three-dimensional real-time in vivo magnetic particle imaging. Phys. Med. Biol. 54: L1–L10.
Wilming, N., Murphy, P.R., Meyniel, F. et al. (2020). Large-scale dynamics of perceptual decision information across human cortex. Nat Commun 11, 5109. https://doi.org/10.1038/s41467-020-18826-6
-> Neu: Wu, Y., Han, X., Su, Y. et al. Multiview confocal super-resolution microscopy. Nature (2021).
Foto: Paul Wicks.
2. Nicht-invasive Gehirn-Computer-Schnittstellen
-> Neu: Kraus D, Naros G, Guggenberger R, Leão MT, Ziemann U, Gharabaghi A. (2018):Recruitment of additional corticospinal pathways in the human brain with state-dependent paired associative stimulation. J Neurosci. 2893-17.2017
Kraus D, Naros G, Bauer R, Leão MT, Ziemann U, Gharabaghi A. (2016): Brain-robot interface driven plasticity: Distributed modulation of corticospinal excitability. Neuroimage. 125: 522-532.
Herff, C, Heger, D, de Pesters, A, Telaar, D, Brunner, P, Schalk, G & Schultz, T. (2015): Brain-to-text: decoding spoken phrases from phone representations in the brain. Front. Neurosci. http://dx.doi.org/10.3389/fnins.2015.00217
-> Neu: Nann, M., Cohen, L. G., Deecke L. & Soekadar, S. R. (2019). To jump or not to jump – The Bereitschaftspotential required to jump into 192-meter abyss. Sci Rep. 9: 2243. doi: 10.1038/s41598-018-38447-w.
Quandt, F., Reichert, C., Hinrichs, H., Heinze, H. J., Knight, R. T., & Rieger, J. W. (2012): Single trial discrimination of individual finger movements on one hand: a combined MEG and EEG study. Neuroimage., 59/4: 3316-3324.
Waldert, S., Preissl, H., Demandt, E., Braun, C., Birbaumer, N., Aertsen, A., & Mehring, C. (2008): Hand movement direction decoded from MEG and EEG. J Neurosci., 28/4: 1000-1008.
3. Nicht-invasive Verfahren zur Hirnstimulierung
Tufail, Y., Matyushov, A., Baldwin, N., Tauchmann, M. L., Georges, J., Yoshihiro, A., Tillery, S. I., & Tyler, W. J. (2010): Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron, 66/5: 681-694.
Foto: Sven Hoppe, Fotolia.com
4. Human-spezifische Krankheitsmodelle (Diseases-in-a-dish)
Callaway, E. (2011): Cells snag top modelling job. Nature 469/7330: 279.
Se Hoon Choi, Young Hye Kim, Matthias Hebisch, Christopher Sliwinski, Seungkyu Lee, Carla D’Avanzo, Hechao Chen, Basavaraj Hooli, Caroline Asselin, Julien Muffat, Justin B. Klee, Can Zhang, Brian J. Wainger, Michael Peitz, Dora M. Kovacs, Clifford J. Woolf, Steven L. Wagner, Rudolph E. Tanzi & Doo Yeon Kim (2014): A three-dimensional human neural cell culture model of Alzheimer’s disease. doi:10.1038/nature13800
Dekkers, JF, Berkers, G, Kruisselbrink, E, et al. (2016): Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Science 8/344.
Graffmann N, Ring S, Kawala MA, Wruck W, Ncube A, Trompeter HI, et al. (2016): Modelling NAFLD with human pluripotent stem cell derived immature hepatocyte like cells reveals activation of PLIN2 and confirms regulatory functions of PPARalpha. Stem cells and development.
Itzhaki, I., Maizels, L., Huber, I., Zwi-Dantsis, L., Caspi, O., Winterstern, A., Feldman, O., Gepstein, A., Arbel, G., Hammerman, H., Boulos, M., & Gepstein, L. (2011): Modelling the long QT syndrome with induced pluripotent stem cells. Nature, 471/7337: 225-229.
D. Huh, D. C. Leslie, B. D. Matthews, J. P. Fraser, S. Jurek, G. A. Hamilton, K. S. Thorneloe, M. A. McAlexander, D. E. Ingber, A Human Disease Model of Drug Toxicity–Induced Pulmonary Edema in a Lung-on-a-Chip Microdevice. Sci. Transl. Med. 4, 159ra147 (2012).
Jerome Mertens, Apuã C.M. Paquola, Manching Ku, Emily Hatch, Lena Böhnke, Shauheen Ladjevardi, Sean McGrath, Benjamin Campbell, Hyungjun Lee, Joseph R. Herdy, J. Tiago Goncalves, Tomohisa Toda, Yongsung Kim, Jürgen Winkler, Jun Yao, Martin Hetzer, and Fred H. Gage (2015): Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell 17, 1-14. DOI: http://dx.doi.org/10.1016/j.stem.2015.09.001
Moretti, A., Bellin, M., Welling, A., Jung, C.B., Lam, J.T., Bott-Flugel, L., Dorn, T., Goedel, A., Hohnke, C., Hofmann, F., Seyfarth, M., Sinnecker, D., Schomig, A., & Laugwitz, K.L. (2010): Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl. J Med 363/15: 1397-1409.
Nguyen, D.-H. T., Stapleton, S. C., Yang, M. T., Cha, S. S., Choi, C. K., Galie, P. A. & Chen, C. S. (2013): Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. PNAS 110/17: 6712-6717.
-> Neu: Christos Papadimitriou, Hilal Celikkaya, Mehmet I. Cosacak, Violeta Mashkaryan, Laura Bray, Prabesh Bhattarai, Kerstin Brandt, Heike Hollak, Xin Chen, Shuijin He, Christopher L. Antos, Weilin Lin, Alvin Kuriakose Thomas, Andreas Dahl, Thomas Kurth, Jens Friedrichs, Yixin Zhang, Uwe Freudenberg, Carsten Werner & Caghan Kizil (2018). 3D Culture Method for Alzheimer’s Disease Modeling Reveals Interleukin-4 Rescues Ab42-Induced Loss of Human Neural Stem Cell Plasticity. Developmental Cell 46: 85–101. https://doi.org/10.1016/j.devcel.2018.06.005.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007): Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131/5: 861-872.
Wang, G., McCain, M., et. al. (2014): Modeling the mitochondrial cardiomyopathy of Barth syndrome with iPSC and heart-on-a-chip technologies. Nature Medicine.
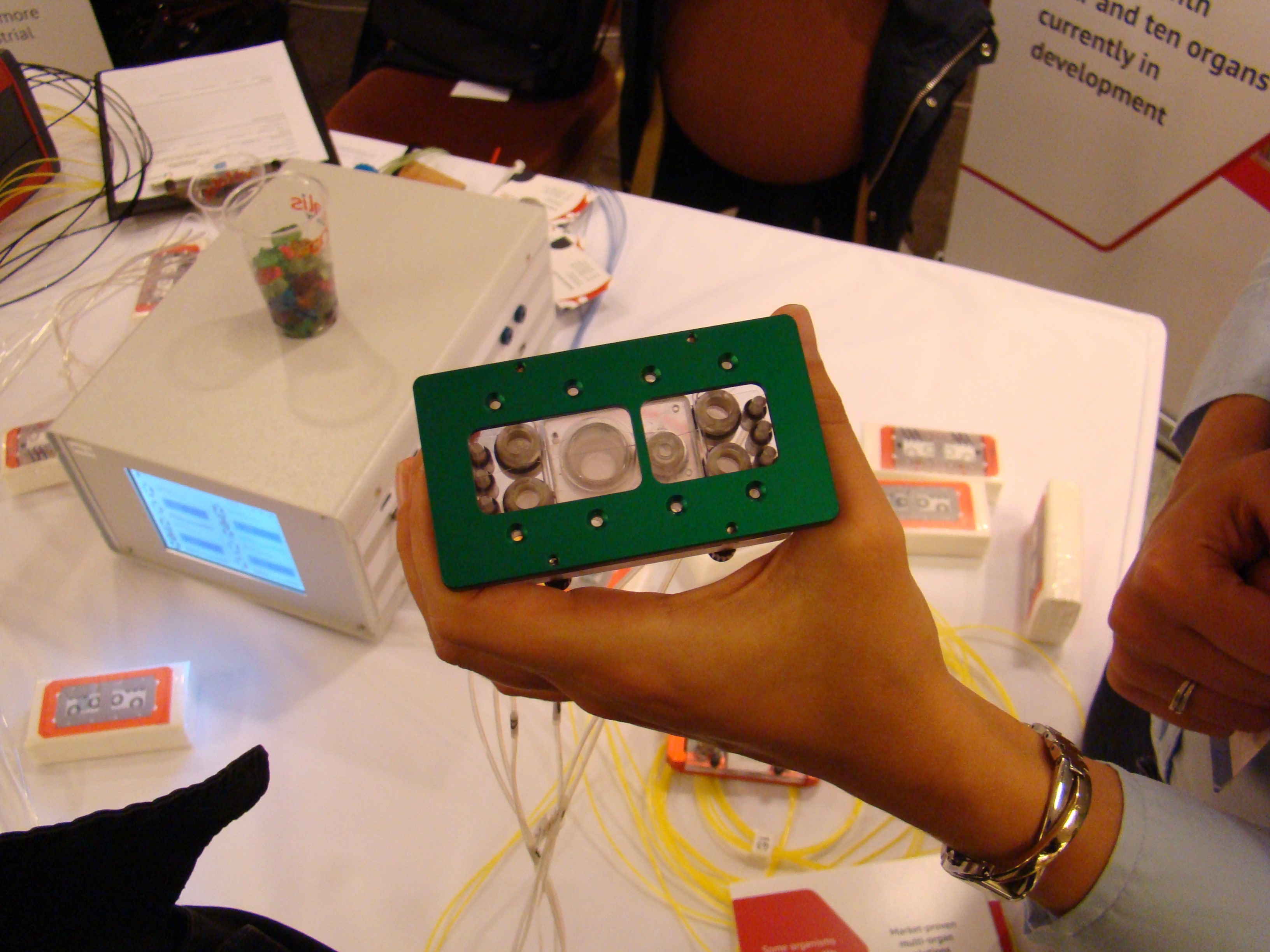
5. Mikrofludische Systeme
-> Neu: Chen, H.J., Miller, P. & Shuler, M. L. (2018). A pumpless body-on-a-chip model using a primary culture of human intestinal cells and a 3D culture of liver cells. Lab Chip. 2018 Jun 8. doi: 10.1039/c8lc00111a.
Huh, D., Matthews, B. D., Mammoto, A., Montoya-Zavala, M., Hsin, H.Y., & Ingber, D. E. (2010): Reconstituting organ-level lung functions on a chip. Science, 328/5986: 1662-1668.
Huh, D., Hamilton, G. A., & Ingber, D. E. (2011): From 3D cell culture to organs-on-chips. Trends Cell Biol, 21/12: 745-754.
Neuzil, P. et al. (2012): Revisiting lab-on-a-chip technology for drug discovery. Nat Rev Drug Discov. 11: 620 - 32.
Friedrich Schuler, Frank Schwemmer, Martin Trotter, Simon Wadle, Roland Zengerle, Felix von Stetten und Nils Paust (2015): Centrifugal step emulsification applied for absolute quantification of nucleic acids by digital droplet RPA. Lab Chip, 2015,15, 2759-2766. DOI: 10.1039/C5LC00291E
Tsai, M., Kita, A., Leach, J., Rounsevell, R., Huang, J. N., Moake, J., Ware, R. E., Fletcher, D. A., & Lam, W. A. (2012): In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest, 122/1: 408-418.






 Dr. rer. nat.
Dr. rer. nat. Menschen für Tierrechte - Tierversuchsgegner Rheinland-Pfalz e.V.
Menschen für Tierrechte - Tierversuchsgegner Rheinland-Pfalz e.V.